Abstract
Introduction The primary treatment of acute graft-versus-host disease (GVHD), prolonged courses of high dose systemic corticosteroids (SCS), cause significant morbidities, including an increased risk of serious infections. We used validated clinical (Minnesota standard [Minn std] risk) and biomarker (Ann Arbor [AA] 1) criteria to identify a group of patients with GVHD that is low risk (LR) for treatment failure and non-relapse mortality (NRM) and who might benefit from less toxic alternatives to SCS. Itacitinib (ita), a novel JAK1 inhibitor, blocks the inflammatory cytokine pathways key to target organ damage without the myelosuppression of JAK1/2 inhibitors such as ruxolitinib. We tested the hypothesis that ita monotherapy would be a safe and effective primary treatment of low risk GVHD in a multicenter phase II trial and compared outcomes to a matched control cohort of MAGIC pts with LR GVHD treated with SCS.
Methods From February 2019 to April 2021, 70 pts with LR GVHD (Minn std risk/AA1) GVHD were treated with ita 200mg/day for 28 days; responders were allowed a second 28 day cycle. Ita was discontinued without tapering. Biomarker scores were available within 30 hours from sample collections, and treatment could be delayed up to 4 days after screening to accommodate a mostly outpatient population. A contemporaneous cohort of 140 control pts, matched for key characteristics, including target organ severity, donor type, HLA-match, and GVHD prophylaxis, was created from the Mount Sinai Acute GVHD International Consortium (MAGIC) database and biorepository. Control pts were treated with standard doses of SCS (median starting dose 1.1 mg/kg). ORR at 28 days of treatment was defined as the proportion of pts achieving complete or partial response of GVHD symptoms without additional systemic therapy. Infections required laboratory/imaging proof and were considered serious if they required systemic treatment.
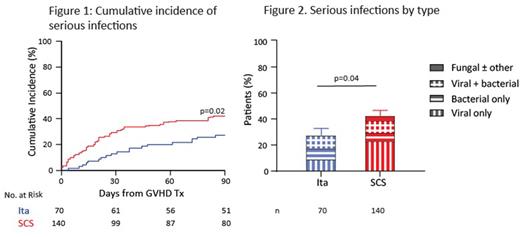
Results Both ita and SCS treatment produced excellent ORR (ita: 89%; SCS: 86%; p=0.67) including in pts with lower GI GVHD (86% vs 86%, p=1). Similarly high ORR was observed for both cohorts for all subgroup comparisons including by GVHD grade, target organ involvement, age, donor type, HLA-match, conditioning regimen, GVHD prophylaxis, and number of days post-BMT to treatment initiation. ORR was unaffected by the time from screening to initiation of ita (≤48 hours: 90%; >48 hours: 87%; p=1). Significantly more patients treated with itacitinib achieved a response by day 7 compared to those treated with SCS (81% vs 66%, p=0.02) and among responders, ORR were equally durable up to day 90 in both groups (89% vs 88%; p=1). There were no significant differences between ita and SCS pts for 1-year relapse (18% vs 21%, p=0.64), chronic GVHD (28% vs 32%, p=0.33), NRM (4% vs 11%, p=0.21), or survival (88% vs 80%, p=0.11). Regarding the safety data of ita monotherapy, the incidence of grade ≥3 non-hematologic and non-infectious TEAEs was similar to the SCS cohort with no event occurring in >10% of pts. The incidence of new or worsening grade ≥3 neutropenia, anemia, or thrombocytopenia was similar between ita and SCS cohorts. In fact, grade ≥3 leukopenia occurred less frequently in the ita group (16% vs 31%, p=0.02). Ita treatment resulted in an 91% lower cumulative steroid dose through day 28 compared to SCS (1.9 vs 22.0 mg/kg, p<0.001) and fewer pts developed at least one serious infection within 90 days (27% vs 42%, p=0.02; Fig 1). The mean number of infections per pt was also lower (0.43 vs 0.66; rate ratio 0.65 [0.43-0.97]), as was the likelihood of developing a severe (BMT CTN grade 2 or 3) infection (odds ratio 0.50 [95% CI: 0.27, 0.93]). The reduction in serious infections was driven by fewer viral infections in the ita group (Fig 2), particularly in the first 28 days and not explained by differences in letermovir use.
Conclusion A short course of itacitinib monotherapy produced very high response rates that occurred faster than treatment with SCS, were equally durable and with a similar risk for cGVHD in patients treated for LR GVHD. Importantly, ita treatment resulted in significantly less severe leukopenia and a significantly lower incidence of serious infections than SCS, likely a result of dramatically less exposure to systemic steroids. Ita monotherapy should be further studied as primary treatment for LR GVHD.
Disclosures
Etra:Kadmon: Consultancy. Alousi:Genetech: Consultancy; Prolacta: Consultancy; Incyte: Honoraria, Research Funding; Sanofi / Kadmon: Honoraria; Mallinkrodt: Honoraria. Al Malki:Hasna Biopharma: Membership on an entity's Board of Directors or advisory committees; Miltenyi Biotec: Consultancy, Research Funding; Incyte: Consultancy, Research Funding; CareDx: Consultancy, Research Funding; Gilead: Consultancy, Research Funding; NexImmune: Consultancy, Research Funding. Choe:Abbvie: Other: independent endpoint review committee for trial. Defilipp:Regimmune: Research Funding; Taiho Oncology: Research Funding; Syndax Pharmaceuticals: Consultancy; Kadmon: Consultancy; Omeros: Consultancy; Incyte: Consultancy; MorphoSys: Consultancy; Incyte: Research Funding. Kitko:Horizon Therapeutics: Consultancy. Ayuk:Mallinckrodt/Therakos: Research Funding; Celgene/BMS: Consultancy; Gilead: Consultancy; Janssen: Consultancy; Mallinckrodt/Therakos: Consultancy; Medac: Consultancy; Miltenyi Biomedicine: Consultancy; Novartis: Consultancy; Takeda: Consultancy. Hexner:Samus Therapeutics, Novartis Oncology: Research Funding; Blueprint Medicines Corporation: Consultancy, Research Funding; American Board of Internal Medicine: Other: Member of the hematology exam committee; Tmunity Therapeutics: Research Funding; PharmaEssentia: Consultancy. Ozbek:Eli Lilly and Company: Current Employment. Qayed:Vertex: Honoraria; Novartis: Honoraria. Reshef:Precision Biosciences: Research Funding; Shire: Research Funding; Takeda: Research Funding; Bristol-Myers Squibb: Consultancy; Jasper: Consultancy; Novartis: Consultancy; Regeneron: Consultancy; Synthekine: Consultancy; TScan: Consultancy; Bayer: Consultancy; Kite: Consultancy, Research Funding; Magneta: Consultancy; Pfizer: Consultancy; Celgene: Research Funding; Pharmacyclics: Consultancy, Research Funding; Incyte: Consultancy, Research Funding; Immatics: Research Funding; J&J: Research Funding; Gilead Science: Consultancy, Research Funding; Atara Biotherapeutics: Consultancy, Research Funding. Chen:Jasper: Consultancy; Incyte: Consultancy; Gamida Cell: Consultancy; Equillium: Consultancy; Celularity: Consultancy; Actimium: Consultancy; Novartis: Consultancy. Ferrara:Physicians Education Resource: Consultancy; Nimbus Discovery: Consultancy; Eurofins Viracor: Consultancy, Patents & Royalties; Bo Fu Rui: Consultancy; Alexion: Consultancy; Mesoblast: Research Funding; Incyte: Research Funding; Genentech: Research Funding; Equillium: Consultancy, Research Funding; Xenikos: Consultancy. Levine:Eurofins Viracor: Patents & Royalties; X4 Pharmaceuticals: Consultancy; Mallinckrodt/Therakos: Consultancy; Jazz: Consultancy; Bluebird Bio: Consultancy; Mesoblast: Consultancy, Research Funding; MaaT Pharma: Research Funding; Incyte: Research Funding; Equillium: Consultancy, Research Funding; Biogen: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal